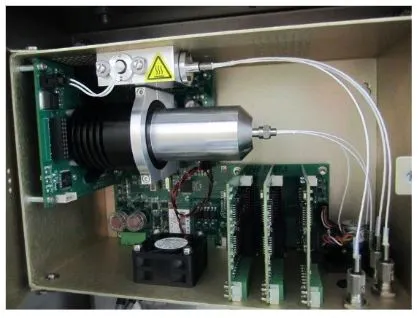
Have you ever sipped into your Romex Coveralls and walked the plant floor to locate an analyzer that needs servicing, only to open the access panel on the analyzer, and find a mess of wires and fiber optic cables?
Fiber Optic Cables Part of Total NIR or UV-VIS Analytical System
A complete Guided Wave system has three main components: 1) the analyzer, 2) the fiber optic cables, and 3) the sample interface. Optical grade fiber cables are used to carry the light from the analyzer to the sample and back. Using high performance fiber cables permits the sample interface to be located 100 meters, or more in some cases, from the analyzer for safety or convenience.
Additional Meter of Fiber Cable Allows for Easier Service and Repair
Guided Wave recommends an additional meter of fiber cable to allow for service and repair. Repairing a broken fiber termination is much preferred to replacing the entire cable. The Fiber Junction Box provides safe storage for this fiber cable. Consider installing a fiber junction box next to your NIRO analyzer.